Implementing Global Health Regulations in the Age of Emerging Biotechnologies
The Necessity of Global Health Regulations
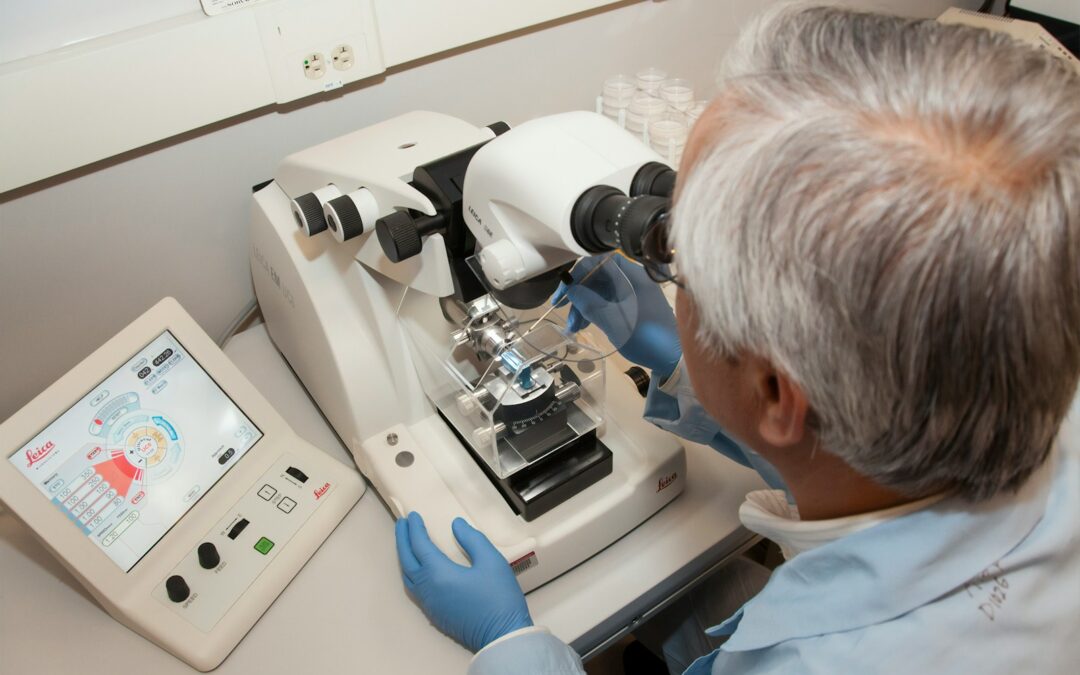
The advent of emerging biotechnologies has revolutionized the field of medicine, offering unprecedented opportunities for the development of new medical treatments. However, with these advancements comes the crucial need for comprehensive global health regulations to ensure their safe and equitable use. Effective regulations are vital to mitigate potential risks associated with new biotechnologies, such as unintended side effects, ethical concerns, and unequal access to treatments.
In regions like Saudi Arabia and the UAE, where healthcare innovation is rapidly advancing, the implementation of global health regulations is particularly important. These countries are at the forefront of adopting cutting-edge medical technologies, including AI-driven diagnostics, gene editing, and personalized medicine. By adhering to international health standards, they can ensure that the benefits of these technologies are maximized while minimizing potential harms.
Moreover, global health regulations play a crucial role in fostering public trust in emerging biotechnologies. When people are confident that new medical treatments are thoroughly tested and regulated, they are more likely to embrace and benefit from these innovations. This trust is essential for the widespread adoption and success of new medical technologies, ultimately leading to improved health outcomes on a global scale.
Addressing Ethical and Safety Concerns
One of the primary functions of global health regulations is to address the ethical and safety concerns associated with emerging biotechnologies. Innovations such as CRISPR gene editing and AI-powered healthcare solutions present complex ethical dilemmas that require careful consideration. Regulations must ensure that these technologies are developed and used in ways that respect human rights, privacy, and the principles of equity and justice.
In Dubai and Riyadh, regulatory bodies are working diligently to establish frameworks that address these ethical concerns while promoting innovation. For instance, regulations governing the use of AI in healthcare must ensure transparency, accountability, and the protection of patient data. Similarly, the ethical implications of gene editing, such as potential genetic discrimination or unintended genetic modifications, must be thoroughly examined and addressed.
Safety is another critical aspect that global health regulations must cover. Emerging biotechnologies often involve new procedures and treatments that have not been widely tested over long periods. Regulatory bodies must ensure rigorous testing and evaluation of these technologies to identify and mitigate any potential risks. This includes conducting extensive clinical trials, monitoring long-term effects, and establishing protocols for the safe implementation of new treatments.
Ensuring Equitable Access to Medical Innovations
Another key objective of global health regulations is to ensure equitable access to emerging biotechnologies. The benefits of new medical treatments should not be limited to certain populations or regions but should be accessible to all who need them. This is particularly important in the context of global health, where disparities in access to healthcare can exacerbate existing inequalities.
In Saudi Arabia and the UAE, efforts are being made to bridge the gap in access to advanced medical treatments. By collaborating with international health organizations and adhering to global health regulations, these countries can ensure that their populations benefit from the latest medical innovations. This includes not only providing access to cutting-edge treatments but also ensuring that healthcare professionals are adequately trained to use these technologies effectively.
Furthermore, global health regulations can facilitate the equitable distribution of resources and technologies. For instance, in the case of a global health crisis, such as a pandemic, regulations can ensure that vaccines and treatments are distributed fairly and efficiently. This is essential for controlling the spread of diseases and ensuring that all countries, regardless of their economic status, have access to life-saving medical treatments.
Conclusion
The implementation of global health regulations for emerging biotechnologies is essential for ensuring the safe and equitable use of new medical treatments. As advancements in biotechnologies continue to revolutionize healthcare, robust regulatory frameworks are needed to address ethical and safety concerns, build public trust, and ensure equitable access to medical innovations. In regions like Saudi Arabia and the UAE, these regulations play a crucial role in fostering innovation while protecting public health. By adhering to international health standards and collaborating with global health organizations, these countries can lead the way in the safe and equitable implementation of emerging biotechnologies, ultimately improving health outcomes on a global scale.
#GlobalHealthRegulations #EmergingBiotechnologies #MedicalTreatments #HealthcareInnovation #SaudiArabia #UAE #Riyadh #Dubai #ArtificialIntelligence #Blockchain #TheMetaverse #ExecutiveCoaching #GenerativeAI #ModernTechnology #BusinessSuccess #LeadershipSkills #ProjectManagement